Publication 2008


▼Pyridinium 1,1'-Binaphthyl-2,2'-disulfonates as Highly Effective Chiral Bronsted Acid-Base Combined Salt Catalysts for Enantioselective Mannich-Type Reaction
Manabu Hatano
Toshikatsu Maki
Katsuhiko Moriyama
Manabu Arinobe
Kazuaki Ishihara*
J. Am. Chem. Soc. 2008, 130, 16858-16860.
We have established, for the first time, a practical synthesis of chiral 1,1'-binaphthyl-2,2'-disulfonic acid (BINSA, 1) from inexpensive BINOL. An efficient enantioselective catalysis in the Mannich-type reactions of diketones and ketoester equivalents with aldimines was developed using chiral 1-achiral 2,6-diarylpyridine (2) combined salts, which acted as convenient chiral tailor-made Bronsted acid-base organocatalysts in situ.

▼Design of High-Performance Catalysts Based on Acid-Base Combined Salts and Development of Highly Selective Reactions
Akira Sakakura*
J. Synth. Org. Chem., Jpn. 2008, 66(10), 942-952.
The design of highly functionalized artificial catalysts is very important for practical organic synthesis. We have succeeded in the rational design of high-performance catalysts based on acid-base combined salts. Acid-base combined salt catalysts can be classified into three types: (1) Metal oxide-Lewis base combined salts, (2) Bronsted acid-Bronsted base combined salts, and (3) Lewis acid-Lewis base combined salts. Here we report our recent studies on development of highly selective cyclization reactions for the synthesis of key intermediates of bioactive natural products. Molybdenum(VI) oxide-bis(quinolinolato) complexes (class 1) efficiently catalyze biomimetic dehydrative cyclization of serine, threonine and cysteine derivatives to give oxazolines and thiazolines. Chiral 1, 1'-binaphthyl-2, 2'-diammonium salts (class 2) are useful catalysts for enantioselective Diels-Alder reaction of α-acyloxyacroleins. We also have developed the first enantioselective biomimetic iodocyclization of isoprenoids using a chiral nucleophilic promoter (class 3).
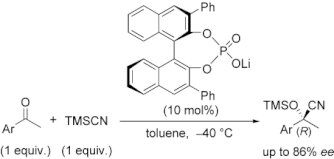
▼Chiral Lithium Salts of Phosphoric Acids as Lewis Acid-Base Conjugate Catalysts for the Enantioselective Cyanosilylation of Ketones
Manabu Hatano
Takumi Ikeno
Tokihiko Matsumura
Shinobu Torii
Kazuaki Ishihara*
Adv. Synth. Catal. 2008, 350, 1776-1780.
The catalytic enantioselective cyanosilylation of aromatic ketones was developed by using chiral lithium salts of (R)-BINOL- or (S)-BINAM-derived phosphoric acid compounds. In the presence of 10 mol% of chiral conjugate lithium salts, the corresponding tertiary cyanohydrins were obtained in high yields with moderate to high enantioselectivities. This is the first efficient example of asymmetric catalysis using lithium salts of synthetically useful chiral phosphoric acid compounds. A possible catalytic mechanism and transition states are also discussed as a preliminary working hypothesis.

▼Open-Air and Solvent-Free Ester Condensation Catalyzed by Sulfonic Acids
Akira Sakakura
Yoshiki Koshikari
Kazuaki Ishihara*
Tetrahedron Lett. 2008, 49, 5017-5020.
▷http://dx.doi.org/10.1016/j.tetlet.2008.06.058
Under open-air and solvent-free conditions, catalytic amounts of sulfonic acids efficiently promote ester condensation between an equimolar mixture of carboxylic acids and alcohols. Since the present protocol does not require solvents under open-air conditions, a large amount of esters can be synthesized in a rather small apparatus.
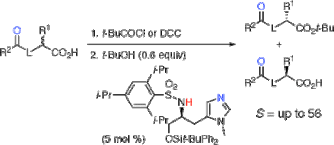
▼Kinetic Resolution of Racemic Carboxylic Acids by an L-Histidine-Derived Sulfonamide-Induced Enantioselective Esterification Reaction
Kazuaki Ishihara*
Yuji Kosugi
Shuhei Umemura
Akira Sakakura
Org. Lett. 2008, 10, 3191-3194.
The direct and catalytic kinetic resolution of racemic carboxylic acids bearing a Bronsted base such as O-protected α-hydroxy carboxylic acids and N-protected α-amino acids has been accomplished through an L-histidine-derived sulfonamide-induced enantioselective esterification reaction with tert-butyl alcohol for the first time. Highly asymmetric induction [S(kfast/kslow) = up to 56] has been achieved under the equilibrium between a chiral catalyst and two diastereomeric acylammonium salts through an intramolecular hydrogen-bonding interaction.

▼Catalytic Enantioselective Organozinc Addition toward Optically Active Tertiary Alcohol Synthesis
Manabu Hatano
Kazuaki Ishihara*
Chem. Record 2008, 8, 143-155.
A highly enantioselective organozinc (R2Zn) addition to a series of aldehydes and ketones was developed based on conjugate Lewis acid-Lewis base catalysis. Optically active secondary and tertiary alcohols were obtained in high yields with high enantioselectivities without Ti(IV) compounds. Bifunctional chiral 3,3-diphosphoryl-BINOL ligands were designed and prepared through a phospho-Fries rearrangement as a key step. On the other hand, bifunctional chiral phosphoramide ligands were designed and prepared from L-valine. Mechanistic studies were performed by X-ray analyses of Zn(II) cluster and chiral ligands, a 31P NMR experiment on Zn(II) complexes, and stoichiometric reactions with some chiral or achiral Zn(II) complexes to propose a transition state assembly that includes monomeric active intermediates.

▼Development of Highly Efficient Acid-Base Combination Catalyses Based on Carbon-Carbon Bonds Activation in Organometallic Reagents
Manabu Hatano
Kazuaki Ishihara*
J. Synth. Org. Chem., Jpn. 2008, 66(6), 564-577.
▷http://dx.doi.org/10.5059/yukigoseikyokaishi.66.564
For synthesis of secondary and tertiary alcohols, practical carbon-carbon bond forming reactions between organometallic reagents and carbonyl compounds have been developed over a century since the discovery of Grignard reaction in 1900. Traditional organometallic reagents (i.e. Li, Mg, Si, Cu, and Zn reagents) should involve some fundamental functions, such as alkylating reagents, Lewis acids, and Bronsted bases. Thus, in principle, the efficiency of the nucleophilic addition should significantly increase if a carbon-metal bond in organometallic reagents can be activated by Lewis base catalysts and the substrates can be activated by native Lewis acid function of organometallic reagents. We report here that the highly efficient catalytic carbon-carbon bond forming reactions via carbon-metal bond activation in organometallic reagents using simple metal salt catalysts. A variety of acid-base promoting catalytic reactions, such as Grignard reaction, organozinc addition, 1,4-addition of organocopper reagents, trimethylsilylcyanation, and Mukaiyama aldol reaction, were achieved toward the efficient asymmetric catalyses.
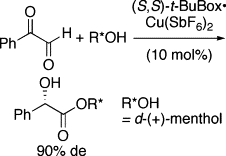
▼Asymmetric Intramolecular Cannizzaro Reaction of Anhydrous Phenylglyoxal
Kazuaki Ishihara*
Takayuki Yano
Makoto Fushimi
J. Fluorine Chem. 2008, 129, 994-997.
▷http://dx.doi.org/10.1016/j.jfluchem.2008.04.008
Double asymmetric induction was achieved in the reaction of anhydrous phenylglyoxal with d-(+)-menthol promoted by a (S,S)-t-BuBox・copper(II) hexafluoroantimonate complex.
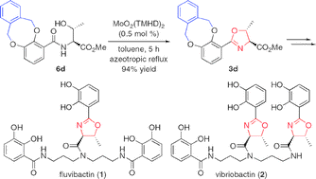
▼Convergent Total Syntheses of Fluvibactin and Vibriobactin Using Molybdenum(VI) Oxide-Catalyzed Dehydrative Cyclization as a Key Step
Akira Sakakura
Shuhei Umemura
Kazuaki Ishihara*
Chem. Commun. 2008, 3561-3563.
Efficient total syntheses of fluvibactin and vibriobactin have been achieved via molybdenum(VI) oxide-catalyzed dehydrative cyclization, Sb(OEt)3-catalyzed ester-amide transformation, and WSCI and HOAt-promoted dehydrative amide formation.

▼Organocatalytic Enantioselective Diels-Alder Reaction of Dienes with α-(N,N-Diacylamino)acroleins
Kazuaki Ishihara*
Kazuhiko Nakano
Matsujiro Akakura
Org. Lett. 2008, 10(13), 2893-2896.
Catalytic and highly enantioselective Diels-Alder reaction of cyclic and acyclic dienes with α-phthalimidoacroleins provides cyclic α-quaternary α-amino acid precursors. The conformationally flexible chiral ammonium salt of H-L-Phe-L-Leu-N(CH2CH2)2-reduced triamine with pentafluorobenzensulfonic acid is very effective as an asymmetric catalyst for the Diels-Alder reaction.
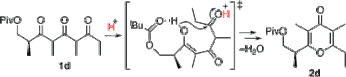
▼Rate-Accelerating Effect by the Neighboring-Group Participation of Protecting Groups in the Dehydrative Cyclization of 1,3,5-Triketones
Akira Sakakura
Hitoshi Watanabe
Kazuaki Ishihara*
Org. Lett. 2008, 10, 2569-2572.
Neighboring-group participation of an acyl protecting group efficiently promotes the Bronsted acid-catalyzed dehydrative cyclization of 1,3,5-triketones to γ-pyrones, whereas a bulky silyloxy group in the β-position retards the cyclization. This reaction provides an efficient synthetic route for a common intermediate for the synthesis of γ-pyrone-containing bioactive natural products.
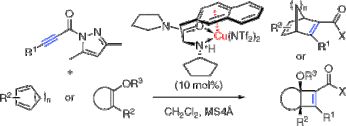
▼Catalytic Enantioselective [2 + 4] and [2 + 2] Cycloaddition Reactions with Propiolamides
Kazuaki Ishihara*
Makoto Fushimi
J. Am. Chem. Soc. 2008, 130(24), 7532-7533.
We report here the catalytic and highly enantioselective [2 + 4] and [2 + 2] cycloaddition reactions of electron-rich dienes or silyl enol ethers with electron-deficient propiolamide derivatives induced by copper(II)-3-(2-naphthyl)-L-alanine amide complex.

▼Zwitterionic Salts as Mild Organocatalysts for Transesterification
Kazuaki Ishihara*
Masatoshi Niwa
Yuji Kosugi
Org. Lett. 2008, 10, 2187-2190.
The exothermic reaction of 3,5-bis(trifluoromethyl)phenyl or 4-nitrophenyl isothiocyanate with 4-pyrrolidinopyridine (PPY) gave the corresponding arylaminothiocarbonylpyridinium salts in quantitative yields. These novel zwitterionic salts were effective as organocatalysts for the transesterification reaction of an equimolar mixture of methyl carboxylates and alcohols in hydrocarbons such as heptane and octane under azeotropic reflux conditions with the removal of methanol. In sharp contrast, PPY was inert as a catalyst under the same reaction conditions.
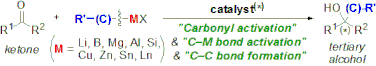
▼Recent Progress in the Catalytic Synthesis of Tertiary Alcohols from Ketones with Organometallic Reagents
Manabu Hatano
Kazuaki Ishihara*
Synthesis 2008, 1647-1675.
Efficient tertiary alcohol synthesis is currently one of the most rapidly advancing fields in organic chemistry, and recent powerful catalytic methodologies can provide versatile tertiary alcohols, even with enantioselective properties. This review summarizes work on the highly efficient catalytic synthesis of tertiary alcohols from ketones and organometallic reagents. New trends in the catalytic synthesis of tertiary alcohols, including functionalized ones, such as tertiary cyanohydrins and tertiary aldols, are examined from the frontier viewpoints that use typical or transition-metal catalysts, organocatalysts, and metal ate complexes, among others.
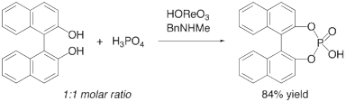
▼Selective Synthesis of Cyclic Phosphoric Acid Derivatives Through Oxorhenium(VII)-Catalyzed Dehydrative Condansation of Phosphoric Acid Alcohols
Akira Sakakura
Masayuki Sakuma
Mikimoto Katsukawa
Kazuaki Ishihara*
Heterocycles 2008, 76, 657-665.
The selective synthesis of phosphoric acid diesters has been achieved through the direct catalytic dehydrative condensation of phosphoric acid with two equivalents of alcohols. The present method works especially well for the synthesis of cyclic phosphoric acid diesters. The combination of perrhenic acid and N-methylbenzylamine efficiently catalyzes the dehydrative condensation of phosphoric acid with equimolar amounts of diols to give cyclic phosphoric acid diesters in excellent yields.

▼Enantioselective Alkynylation to Aldimines Catalyzed by Chiral 2,2’-Di(2-Aminoaryloxy)-1,1’-Binaphthyl-Copper(I) Complexes
Manabu Hatano
Takafumi Asai
Kazuaki Ishihara*
Tetrahedron Lett. 2008, 49, 379-382.
▷http://dx.doi.org/10.1016/j.tetlet.2007.11.032
The enantioselective alkynylation of aldimines with terminal acetylenes catalyzed by chiral Cu(I) complexes with (R)-2,2’-di(2-aminoaryloxy)-1,1’-binaphthyl ligands (7) was examined. Chiral C2-symmetric N,N-ligands 7, which have primary aniline moieties, were readily prepared from inexpensive (R)-1,1’-binaphthol (BINOL) as a chiral source. In particular, the reaction of N-benzylidenebenzeneamine 1a with phenylacetylene 2a proceeded smoothly in the presence of 5 mol % of (CuOTf)2・C6H5CH3 and 10 mol % of (R)-7d at room temperature for 24 h, and the corresponding propargylamine 3a was obtained with up to 82% ee.







