Publication 2011


▼Intramolecular Dehydrative Condensation of Dicarboxylic Acids with Bronsted Base-Assisted Boronic Acid Catalysts
Akira Sakakura
Risa Yamashita
Takuro Ohkubo
Matsujiro Akakura
Kazuaki Ishihara*
Aust. J. Chem. 2011, 64, 1458-1465.
▷ http://dx.doi.org/10.1071/CH11301
Bifunctional Bronsted base-assisted boronic acid catalysts, arylboronic acids bearing two sterically bulky (N,Ndialkylamino) methyl groups at the 2,6-positions, exhibit remarkable activities for the dehydrative intramolecular condensation of dicarboxylic acids. The steric bulkiness of the (N,N-dialkylamino)methyl groups of 1, which prevents the formation of less active species such as the N-B chelated species and triarylboroxines 3, is crucial for the high catalytic activity. This is the first successful method for the catalytic dehydrative self-condensation of di- and tetracarboxylic acids.

▼Enantioselective Diels-Alder Reactions with Anomalous endo/exo Selectivities Using Conformationally Flexible Chiral Supramolecular Catalysts
Manabu Hatano
Tomokazu Mizuno
Atsuto Izumiseki
Ryota Usami
Takafumi Asai
Matsujiro Akakura
Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2011, 50(51), 12189-12192.
Swapped selectivities: The use of tailor-made catalysts results in anomalous endo/exo selectivities and high enantioselectivities in the Diels-Alder reactions of cyclopentadiene with different acroleins (see scheme). These supramolecular catalysts are prepared in situ from chiral diols, arylboronic acid, and tris(pentafluorophenyl)borane, and can discriminate the re/si face of the dienophile as well as the endo/exo approach of the diene.

▼Desymmetrization of meso-Glycerol Derivatives Induced by L-Histidine-Derived Acylation Catalysts
Akira Sakakura
Shuhei Umemura
Kazuaki Ishihara*
Adv. Synth. & Catal. 2011, 353(11-12), 1938-1942.
(Article first published online: 10 AUG 2011)
The desymmetrization of meso-glycerol derivatives bearing a 3-pyrroline-1-carbonyl (Pyroc) directing group is demonstrated through an enantioselective acylation reaction promoted by L-histidine-derived bifunctional catalysts. The desired monoacylated products are obtained in good yields (up to 74%) with high enantioselectivities (up to 99% ee).
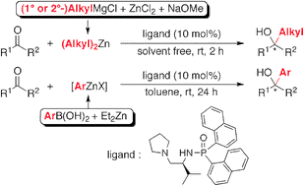
▼Catalytic Enantioselective Alkyl and Aryl Addition to Aldehydes and Ketones with Organozinc Reagents Derived from Alkyl Grignard Reagents or Arylboronic Acids
Manabu Hatano
Riku Gouzu
Tomokazu Mizuno
Hitoshi Abe
Toshihide Yamada
Kazuaki Ishihara*
Catal. Sci. Technol. 2011, 1, 1149-1158.
(Article first published online: 10 AUG 2011)
★Most access ranking: Top 10 (2012.6)
★Most access ranking: 1st. rank (2011.8)
★2011, issue 7 Outside Cover
A highly practical, catalytic enantioselective alkyl and aryl addition to aldehydes and ketones with organozinc reagents, which were prepared in situ from commercially available Grignard reagents or arylboronic acids, was developed. A chiral phosphoramide ligand was essential for promoting the addition reactions in high yields with high enantioselectivities.
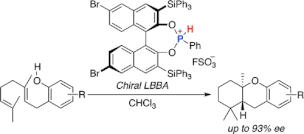
▼Chiral Lewis Base-Assisted Bronsted Acid (LBBA)-Catalyzed Enantioselective Cyclization of 2-Geranylphenols
Akira Sakakura
Masayuki Sakuma
Kazuaki Ishihara*
Org. Lett. 2011, 13, 3130-3133.
Chiral Lewis base-assisted Bronsted acids (Chiral LBBAs) have been designed as new organocatalysts for biomimetic enantioselective cyclization. A salt of a chiral phosphonous acid diester with FSO3H catalyzes the enantioselective cyclization of 2-geranylphenols to give the desired trans-fused cyclized products with high diastereo- and enantioselectivities (up to 98:2 dr and 93% ee).
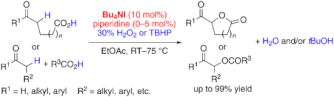
▼In Situ Generated (Hypo)Iodite Catalysts for the Direct alpha-Oxyacylation of Carbonyl Compounds with Carboxylic Acids
Muhammet Uyanik
Daisuke Suzuki
Takeshi Yasui
Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2011, 50(23), 5331-5334.
It's the iodine: The intra- and intermolecular title reaction is catalyzed by an in situ generated ammonium (hypo)iodite species. Either H2O2 or tert-butyl hydroperoxide (TBHP) can be used as an environmentally benign oxidant and a wide range of substrates react to give the corresponding alpha-acyloxycarbonyl compounds in good to excellent yields.

▼Enantioselective Friedel-Crafts Aminoalkylation Catalyzed by Chiral Ammonium 1,1'-Binaphthyl-2,2'-disulfonates
Manabu Hatano
Yoshihiro Sugiura
Matsujiro Akakura
Kazuaki Ishihara*
Synlett 2011, 1247-1250.
A catalytic enantioselective Friedel-Crafts aminoalkylation between aromatic aldimines and N-benzylpyrrole with the use of a homogeneous chiral ammonium salt, (R)-BINSA-N,N-dimethylbutylamine, as a dynamic Bronsted acid-Bronsted base catalyst, is reported. Unlike the results with conventional catalysts, remarkably high reactivity was established at -78 C within 30 minutes, and the corresponding aryl(1H-pyrrol-2-yl)methanamines were obtained with good to high enantioselectivities.

▼Commercially available neat organozincs as highly reactive reagents for catalytic enantioselective addition to ketones and aldehydes under solvent free conditions
Manabu Hatano
Tomokazu Mizuno
Kazuaki Ishihara*
Tetrahedron 2011, 67, 4417-4424.
▷http://dx.doi.org/10.1016/j.tet.2011.02.042
Neat Et2Zn, Ph2Zn, and highly concentrated Me2Zn are highly reactive organozinc reagents, which are commercially available in bulk quantities. We here report a catalytic enantioselective Et2Zn, Ph2Zn, and Me2Zn addition to ketones and aldehydes under solvent free or highly concentrated conditions without Ti(Oi-Pr)4 as a conventional activator of organozinc reagents. The desired optically active tertiary and secondary alcohols were obtained in good yield with high enantioselectivity when compared to the conventional solvent-use conditions. From the viewpoint of ecological and environmental reasons in green chemistry, this catalysis would be practical for not only academic but also industrial use.

▼Brønsted Base-Assisted Boronic Acid Catalysis for the Dehydrative Intramolecular Condensation of Dicarboxylic Acids
Akira Sakakura
Takuro Ohkubo
Risa Yamashita
Matsujiro Akakura
Kazuaki Ishihara*
Org. Lett. 2011, 13, 892-895.
Brønsted base-assisted boronic acid catalysis for the dehydrative self-condensation of carboxylic acids is described. Arylboronic acid bearing bulky (N,N-dialkylamino)methyl groups at the 2,6-positions can catalyze the intramolecular dehydrative condensation of di- and tetracarboxylic acids. This is the first successful method for the catalytic dehydrative self-condensation of carboxylic acids.

▼In situ-generated chiral quaternary ammonium (hypo)iodite catalysis for enantioselective oxidative cyclizations
Muhammet Uyanik
Kazuaki Ishihara*
Chimica Oggi-Chemistry Today 2011, 29(1), 18-21.
▷http://chemistry-today.teknoscienze.com/index.asp
Very recently, we developed the first enantioselective oxidative cycloetherification of ketophenols to 2-acyl- 2,3-dihydrobenzofuran derivatives catalysed by in situgenerated chiral quaternary ammonium (hypo)iodite with hydrogen peroxide or tert-butyl hydroperoxide (TBHP) as an environmentally benign ideal oxidant. The optically active 2-acyl 2,3-dihydrobenzofuran skeleton is a key structure in several medicinally and biologically active compounds.

▼2-Iodoxybenzenesulfonic Acid (IBS) Catalyzed Oxidation of Alcohols
Muhammet Uyanik
Kazuaki Ishihara*
Aldrichmica Acta 2010, 43(3), 83-91.
▷http://www.sigmaaldrich.com/chemistry/chemical-synthesis/learning-center/aldrichimica-acta.html
1. Introduction
2. Hypervalent Iodine Catalysts for Alcohol Oxidation
3. 2-Iodoxybenzenesulfonic Acid (IBS)
3.1. Related Hypervalent Iodine Reagents
3.1.1. HMBI and λ3-Iodane Derivatives
3.1.2. IBS and λ5-Iodane Derivatives
3.2. Discovery of IBS as a Catalyst for Alcohol Oxidation with OxoneR
3.3. Large-Scale Oxidations
3.4. Application to the Oxidative Rearrangement of Tertiary Allylic Alcohols
3.5. Theoretical Calculations and Reaction Mechanism
4. Conclusion
5. Acknowledgement
6. References and Notes
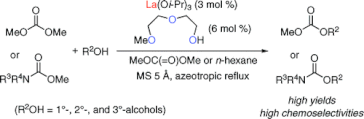
▼Lanthanum(III) Isopropoxide Catalyzed Chemoselective Transesterification of Dimethyl Carbonate and Methyl Carbamates
Manabu Hatano
Sho Kamiya
Katsuhiko Moriyama
Kazuaki Ishihara*
Org. Lett. 2011, 13(3), 430-433.
A practical transesterification of less reactive dimethyl carbonate and much less reactive methyl carbamates with primary (1°), secondary (2°), and tertiary (3°) alcohols was established with the use of a lanthanum(III) complex, which was prepared in situ from lanthanum(III) isopropoxide (3 mol %) and 2-(2-methoxyethoxy)ethanol (6 mol %). In particular, corresponding carbonates and carbamates obtained were of synthetic utility from the viewpoint of the selective protection and/or deprotection of 1°-, 2°-, and 3°-alcohols.
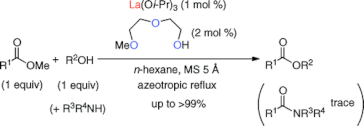
▼Ligand-Assisted Rate Acceleration in Lanthanum(III) Isopropoxide Catalyzed Transesterification of Carboxylic Esters
Manabu Hatano
Yoshiro Furuya
Takumi Shimmura
Katsuhiko Moriyama
Sho Kamiya
Toshikatsu Maki
Kazuaki Ishihara*
Org. Lett. 2011, 13(3), 426-429.
The transesterification of an equimolar mixture of carboxylic esters and primary (1°), secondary (2°), and tertiary (3°) alcohols in hydrocarbon solvents was promoted with high efficiency by a lanthanum(III) complex, which was prepared in situ from lanthanum(III) isopropoxide (1 mol %) and 2-(2-methoxyethoxy)ethanol (2 mol %). The present La(III) catalyst was highly effective for the chemoselective transesterification in the presence of competitive 1°- and 2°-amines. Remarkably, esters were obtained in good to excellent yields as colorless materials without an inconvenient workup procedure.








