Publication 2013

▼Chiral Supramolecular Magnesium(II) Binaphtholate Catalysts for the Enantioselective Direct Mannich-Type Reaction and Hetero-Diels–Alder
Reaction
Manabu Hatano, Takahiro Horibe, Kenji Yamashita, Kazuaki Ishihara*
Asian J. Org. Chem. 2013, 2, 952-956.
(Special Issue: 40 Years of the Mukaiyama Aldol Reaction, 1973–2013)
▷DOI: 10.1002/ajoc.201300190

Just another Mannich Mg: A recent development of chiral supramolecular magnesium(II) binaphtholates has inspired an investigation into two reactions, that is, the direct Mannich-type reaction and the hetero-Diels–Alder reaction, the mechanisms of which require further consideration. The results suggest that chiral di- and trinuclear supramolecular magnesium(II) complexes should play key roles as active catalytic species in these reactions. BINOL=1,1′-bi-2-naphthol.
▼2-ヨードベンゼンスルホン酸(pre-IBS)とOxoneを用いる選択的酸化反応
M. Uyanik、六鹿達矢、石原 一彰、和光純薬時報 2013, 81(4), 5-9.
以下のサイトから和光純薬時報がダウンロードできます。
http://www.wako-chem.co.jp/siyaku/journal/jiho/article/jihoindx.htm#jiho814
尚、この号にはIBS触媒が製品紹介されています。純正化学、Aldrich、TCIに続き、Wakoでも製品化されました。

▼Synthesis of Optically Pure 3,3′-Diaryl Binaphthyl Disulfonic Acids via Stepwise N–S Bond Cleavage
Manabu Hatano, Takuya Ozaki, Keisuke Nishikawa, and Kazuaki Ishihara*
J. Org. Chem. 2013, 78(20), 10405-10413.
DOI:
10.1021/jo401848z
First published online 26 Sep 2013

We developed a practical synthesis of optically pure 3,3′-diaryl-1,1′-binaphthyl-2,2′-disulfonic acids (i.e., (R)- or (S)-3,3′-Ar2-BINSAs) from the parent chiral sulfonimides via stepwise N–S bond cleavage of the sulfonimides and the resultant sulfonamides. This unusual synthesis, which provides arylsulfonic acids from arylsulfonamides, is valuable since common methods particularly give amines with the decomposition of sulfone groups during deprotection.
▼安価なキラル超分子マグネシウム(Ⅱ)-ビナフトラート触媒を用いた光学活性リン化合物の実用的合成法の開発
波多野学、石原 一彰*
月刊ファインケミカル2013, 42(8), 45-52.
http://www.cmcbooks.co.jp/products/detail.php?product_id=4470
筆者らは,Mg(Ⅱ)イオンと安価で入手容易な光学活性ビナフトールを2:3 のモル比で混ぜるだけで自己組織化し,一種類のキラル超分子錯体になることを見いだした。本稿では,光学活性有機リン化合物の新たな精密合成技術として,このキラル超分子マグネシウム(Ⅱ)-ビナフトラート触媒を用いる不飽和カルボニル化合物への有機リン化合物の触媒的不斉求核付加反応を紹介する。
▼“Phosphite–Urea” Cooperative High-Turnover Catalysts for the Highly Selective Bromocyclization of Homogeranylarenes
Yasuhiro Sawamura, Hidefumi Nakatsuji, Akira Sakakura* and Kazuaki Ishihara*
Chem. Sci., 2013, 4(11), 4181-4186.
▷DOI: 10.1039/C3SC51432C
First published online 02 Aug 2013

▼Hydrogen Bonding and Alcohol Effects in Asymmetric Hypervalent Iodine Catalysis: Enantioselective Oxidative Dearomatization of
Phenols
Muhammet Uyanik, Takeshi Yasui, Kazuaki Ishihara*
Angew. Chem. Int. Ed.2013, 52, 9215-9218.
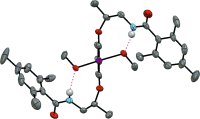
Iodine chooses: A conformationally flexible C2-symmetric organoiodine(III) catalyst for the highly enantioselective catalytic oxidative dearomatization of phenols has been developed. Catalysis is controlled by intramolecular hydrogen-bonding interactions and additional achiral alcohols.
▼Enantioselective Cyanoethoxycarbonylation of Isatins Promoted by a Lewis Base–Brønsted Acid Cooperative Catalyst
Yoshihiro Ogura, Matsujiro Akakura, Akira Sakakura*, Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2013, 52, 8299-8303.
▷DOI: 10.1002/anie.201303572
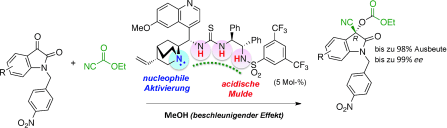
Teaming up to make it happen: In the title reaction, the Lewis basic site of the catalyst activated ethyl cyanoformate, and the deep and flexible Brønsted acidic cavity stabilized and selectively recognized the key reaction intermediate to promote asymmetric acylation (see scheme).
▼Primary Alkylboronic Acids as Highly Active Catalysts for the Dehydrative Amide Condensation of α-Hydroxycarboxylic
Acids
Risa Yamashita, Akira Sakakura*, Kazuaki Ishihara*
Org. Lett. 2013, 15(14), 3654-3657.
▷DOI:10.1021/ol401537f
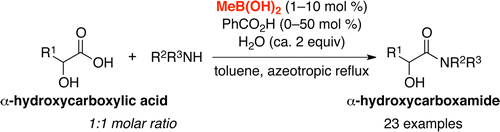
Primary alkylboronic acids such as methylboronic acid and butylboronic acid are highly active catalysts for the dehydrative amide condensation of α-hydroxycarboxylic acids. The catalytic activities of these primary alkylboronic acids are much higher than those of the previously reported arylboronic acids. The present method was easily applied to a large-scale synthesis, and 14 g of an amide was obtained in a single reaction.
▼Kinetic Resolution of Racemic Carboxylic Acids through Asymmetric Protolactonization Promoted by Chiral Phosphonous Acid Diester
Masayuki Sakuma, Akira Sakakura,* and Kazuaki Ishihara*
Org. Lett. 2013, 15(11), 2838-2841.
▷DOI: 10.1021/ol401313d

Chiral phosphonium salts induce the kinetic resolution of racemic α-substituted unsaturated carboxylic acids through asymmetric protolactonization. Both the lactones and the recovered carboxylic acids are obtained with high enantioselectivities and high S (= kfast/kslow) values. Asymmetric protolactonization also leads to the desymmetrization of achiral carboxylic acids. Notably, chiral phosphonous acid diester not only induced the enantioselectivity but also promoted protolactonization.
▼7.7 Product Class 7: Calcium Compounds," in Science of Synthesis Knowledge Updates 2013/1
Manabu Hatano
A. Fuertner (Vol. 9), K. Ishihara (Vo. 7), J. J. Li (Vol. 16), M G. Moloney (Vol. 5) Volume Eds.; Georg Thieme Verlag KG, Stuttgart, 2013.

▼第4章 第6節 縮合剤を用いないカルボン酸誘導体合成技術
石原 一彰*
触媒の設計・反応制御 事例集, pp. 246–254, (株)技術情報協会 (2013年4月30日発刊)
http://www.gijutu.co.jp/doc/b_1715.htm
▼Chiral Magnesium(II) Binaphtholates as Cooperative Brønsted/Lewis Acid–Base Catalysts for the Highly Enantioselective Addition of Phosphorus Nucleophiles to α,β-Unsaturated Esters and Ketones
Manabu Hatano, Takahiro Horibe, Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2013, 52, 4549-4553.

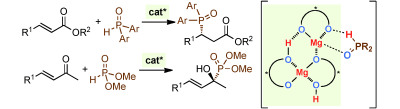
A little cooperation goes a long way: The cooperative Brønsted/Lewis acid–base supramolecular catalysts formed in situ from simple chiral magnesium(II) binaphtholate aqua complexes promoted the highly enantioselective 1,4-hydrophosphinylation of α,β-unsaturated esters with diaryl phosphine oxides and 1,2-hydrophosphonylation of α,β-unsaturated ketones with dialkyl phosphites (see scheme).
Supramolecular catalysts relying on cooperation between the Mg ions and a chelating ligand (represented by carp in the picture) were developed by K. Ishihara and co-workers in their Communication (DOI: 10.1002/anie.201300938). The enantioselective 1,4-hydrophosphinylation of α,β-unsaturated esters with diaryl phosphine oxides and 1,2-hydrophosphonylation of α,β-unsaturated ketones with dialkyl phosphites was achieved using chiral 3:2 complexes of (R)-(H8-)BINOLate/MgII ions.
http://onlinelibrary.wiley.com/doi/10.1002/anie.201302124/abstract
http://onlinelibrary.wiley.com/doi/10.1002/anie.v52.17/issuetoc
▼Baeyer–Villiger Oxidation Using Hydrogen Peroxide
Muhammet Uyanik
Kazuaki Ishihara*
ACS Catalysis 2013, 3, 513-520 (Perspective)

The Baeyer–Villiger (BV) oxidation of carbonyl compounds to the corresponding esters or lactones is one of the most important transformations. We recently introduced a highly efficient and selective LiB(C6F5)4- or Ca[B(C6F5)4]2-catalyzed BV oxidation of ketones with aqueous hydrogen peroxide to give the corresponding lactones in high yield. In this perspective article, we focus on our discovery and the development of BV oxidation reactions and cascade oxidative transformations through representative metal catalysts and organocatalysts.
▼Lanthanum(III) Catalysts for Highly Efficient and Chemoselective Transesterification
Manabu Hatano
Kazuaki Ishihara*
Chem. Commun. 2013, 49, 1983-1997.
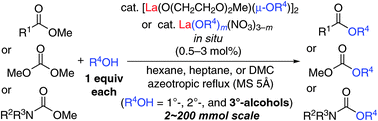
A facile, atom-economical, and chemoselective esterification is crucial in modern organic synthesis, particularly in the areas of pharmaceutical, polymer, and material science. However, a truly practical catalytic transesterification of carboxylic esters with various alcohols has not yet been well established, since, with many conventional catalysts, the substrates are limited to 1°- and cyclic 2°-alcohols. In sharp contrast, if we take advantage of the high catalytic activities of La(Oi-Pr)3, La(OTf)3, and La(NO3)3 as ligand-free catalysts, ligand-assisted or additive-enhanced lanthanum(III) catalysts can be highly effective acid–base combined catalysts in transesterification. A highly active dinuclear La(III) catalyst, which is prepared in situ from lanthanum(III) isopropoxide and 2-(2-methoxyethoxy)ethanol, is effective for the practical transesterification of methyl carboxylates, ethyl acetate, weakly reactive dimethyl carbonate, and much less-reactive methyl carbamates with 1°-, 2°-, and 3°-alcohols. As the second generation, nearly neutral “lanthanum(III) nitrate alkoxide”, namely La(OR)m(NO3)3−m, has been developed. This catalyst is prepared in situ from inexpensive, stable, low-toxic lanthanum(III) nitrate hydrate and methyltrioctylphosphonium methyl carbonate, and is highly useful in the non-epimerized transesterification of α-substituted chiral carboxylic esters, even under azeotropic reflux conditions. In these practical La(III)-catalyzed transesterifications, colorless esters can be obtained in small- to large-scale synthesis without the need for inconvenient work-up or careful purification procedures.








