Publication 2015

▼Book
“19.1.5 有機アルミニウム化合物”
石原一彰
有機合成実験法ハンドブック 第2版(中井武編集代表)、丸善出版、ISBN 978-4-621-08948-4 (2015年11月30日発刊)
http://f.crmf.jp/ntsbookco/cc.php?m=1awz0z390z40a5

▼Enantioselective Cyano-Alkoxycarbonylation of α-Oxoesters Promoted by Brønsted Acid–Lewis Base
Cooperative Catalysts
Kazuaki Ishihara* and Yoshihiro Ogura
Org. Lett. 2015, 17(24), 6070–6073.
DOI: 10.1021/acs.orglett.5b03093
Publication Date (Web): December 4, 2015
http://pubs.acs.org/doi/abs/10.1021/acs.orglett.5b03093
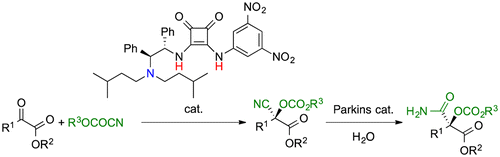
The highly enantioselective cyano-alkoxycarbonylation of α-oxoesters with alkyl cyanoformates is promoted by a new chiral Brønsted acid–Lewis base cooperative organocatalyst. The present
catalysis can be performed at room temperature under nitrogen or air.
▼Boron Tribromide-Assisted Chiral Phosphoric Acid Catalyst for a Highly Enantioselective Diels-Alder Reaction of 1,2-Dihydropyridines
Manabu Hatano, Yuta Goto, Atsuto Izumiseki, Matsujiro Akakura, and Kazuaki Ishihara*
J. Am. Chem. Soc. 2015, 137, 13472-13475.
Publication Date (Web): October 12, 2015 (Communication)
DOI: 10.1021/jacs.5b08693
http://pubs.acs.org/doi/abs/10.1021/jacs.5b08693
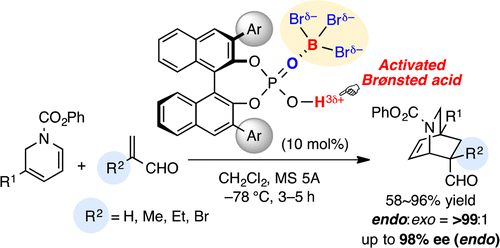
BBr3–chiral phosphoric acid complexes are highly effective and practical Lewis acid-assisted Brønsted acid (LBA) catalysts for promoting the enantioselective Diels–Alder (DA) reaction of α-substituted acroleins and α-CF3 acrylate. In particular, the DA reaction of α-substituted acroleins with 1,2-dihydropyridines gave the corresponding optically active isoquinuclidines with high enantioselectivities. Moreover, transformations to the key intermediates of indole alkaloids, catharanthine and allocatharanthine, are demonstrated.
▼Stereoselective Electrophilic
Cyclization
Akira Sakakura,* Kazuaki Ishihara*
Chem. Rec. 2015, 15(4), 728–742.
▷DOI: 10.1002/tcr.201500005

Electrophilic cyclizations of unactivated alkenes play highly important roles in the synthesis of useful building blocks. This account describes our contributions to the rational design of
monofunctionalized chiral Lewis base catalysts for enantioselective iodo- and protocyclizations. For the stereoselective promotion of electrophilic bromocyclizations, nucleophilic phosphite–urea
cooperative catalysts have been designed.
▼高機能触媒の超分子設計
波多野 学、石原 一彰
化学工業 2015, 66(5), 381-388 (2015年5月1日発行).
▼C- and N-Selective Grignard Addition
Reactions of α-Aldimino Esters in the Presence or Absence of Zinc(II) Chloride: Synthetic Applications to Optically Active Azacycles
Manabu Hatano, Kenji Yamashita,
Kazuaki Ishihara*
Org. Lett. 2015, 17(10), 2412-2415.
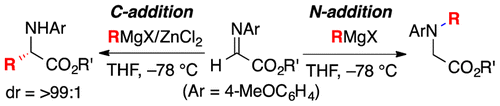
Highly practical synthetic methods were developed for the C- and N-selective Grignard addition reactions of N-4-MeOC6H4-protected α-aldimino esters in the presence or absence of zinc(II)
chloride. Diastereoselective C-alkyl addition, tandem C-alkyl addition–N-alkylation, and some transformations to synthetically useful optically active azacycles were
demonstrated.
▼アルキルZ試薬:亜鉛(Ⅱ)アート錯体を用いるケトン及びイミノエステルへの高効率Grignard付加反応の開発
波多野 学、石原 一彰
和光純薬時報 Vol.83 No.2(2015.04), 2-5.
http://www.wako-chem.co.jp/siyaku/journal/jiho/article/jihoindx.htm#jiho832
参考: アルキルZ試薬にかかわる商品リスト(和光純薬工業)
http://www.wako-chem.co.jp/siyaku/product/chemical/alkylZ/index.htm
▼Boronic Acid-Catalyzed Reactions of Carboxylic Acids, Kazuaki Ishihara, In Topics in
Organometallic Chemistry: Synthesis and Application of Organoboron Compounds, Eds. E. Fernández, A. Whiting, Volume 49, 2015, pp 243–270.
http://link.springer.com/book/10.1007/978-3-319-13054-5
http://link.springer.com/chapter/10.1007/978-3-319-13054-5_8

▼High-Performance Hypoiodite/Hydrogen
Peroxide Catalytic System for the Oxylactonization of Aliphatic γ-Oxocarboxylic Acids
Muhammet Uyanik, Daisuke Suzuki, Mizu Watanabe, Hiroyasu Tanaka, Kikuo Furukawa,
Kazuaki Ishihara*
Chem. Lett. 2015, published on the web Feb 17, 2015

Highly efficient hypoiodite-catalyzed oxylactonization of aliphatic γ-oxocarboxylic acids to the corresponding γ-acyl-γ-butyrolactones was developed. Highly dilute reaction conditions and slow
addition of the oxidant are each highly effective for promoting the high-performance hypoiodite/hydrogen peroxide catalytic oxidation system.
▼Practical Oxidative Dearomatization of
Phenols with Sodium Hypochlorite Pentahydrate
Muhammet Uyanik, Niiha Sasakura, Mitsuyoshi Kuwahata, Yasukazu Ejima, Kazuaki Ishihara*
Chem. Lett. 2015, published on the web Feb 17, 2015

A highly efficient and practical oxidative dearomatization of phenols using sodium hypochlorite pentahydrate as an inexpensive, strong oxidant is reported for the first time. The oxidation
reactions proceeded very rapidly in the presence of water to give the desired products in excellent yields, and sodium chloride and water were the only by-products derived from the
oxidant.
▼Chiral Ammonium Hypoiodite-catalyzed
Enantioselective Oxidative Dearomatization of 1-Naphthols Using Hydrogen Peroxide
Muhammet Uyanik, Niiha Sasakura, Erina Kaneko, Kento Ohori, Kazuaki Ishihara*
Chem. Lett. 2015, 44, 179-181.
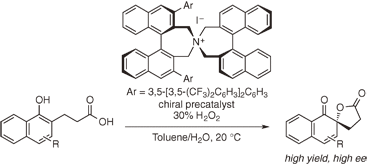
A highly enantioselective oxidative dearomatization of 1-naphthol derivatives (Kita spirolactonization) catalyzed by chiral hypoiodite species prepared in situ from chiral quaternary ammonium
iodide in the presence of hydrogen peroxide is reported.
▼C-Selective and Diastereoselective Alkyl Addition to β,γ-Alkynyl-α-imino Esters with Zinc(II)ate
Complexes
Manabu Hatano, Kenji Yamashita, Mai Mizuno, Orie Ito, Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2015, 54, 2707-2711.
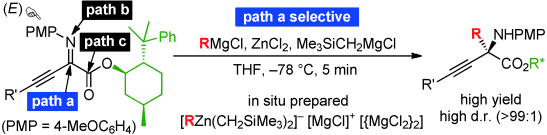

Since umpolung α-imino esters contain three electrophilic centers, regioselective alkyl addition with traditional organometallic reagents has been a serious problem in the practical synthesis of versatile chiral α-amino acid derivatives. An unusual C-alkyl addition to α-imino esters using a Grignard reagent (RMgX)-derived zinc(II)ate was developed. Zinc(II)ate complexes consist of a Lewis acidic [MgX]+ moiety, a nucleophilic [R3Zn]− moiety, and 2 [MgX2]. Therefore, the ionically separated [R3Zn]− selectively attacks the imino carbon atom ,which is most strongly activated by chelation of [MgX]+. In particular, chiral β,γ-alkynyl-α-imino esters can strongly promote highly regio- and diastereoselective C-alkylation because of structural considerations, and the corresponding optically active α-quaternary amino acid derivatives are obtained within 5 minutes in high to excellent yields.











