論文 2014

▼超分子材料の設計と応用展開(監修:原田 明、シーエムシー出版)」
第2章 薬品関係 「3 テーラーメード型超分子触媒(pp. 162-175, 執筆:波多野学、石原一彰)」
http://www.cmcbooks.co.jp/products/detail.php?product_id=4754
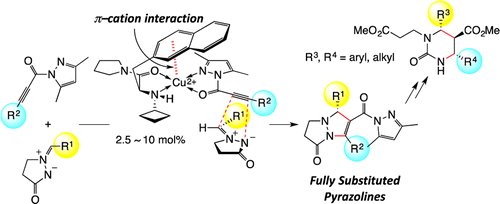
▼Enantioselective 1,3-Dipolar Cycloaddition of Azomethine Imines with Propioloylpyrazoles Induced by Chiral π–Cation Catalysts
Masahiro
Hori, Akira Sakakura*, Kazuaki Ishihara*
J. Am. Chem. Soc. 2014, 136(38),
13198–13201. Published online 16 Sep 2014.

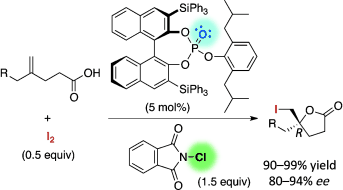
▼High-turnover hypoiodite catalysis for asymmetric synthesis of tocopherols
Muhammet Uyanik, Hiroki Hayashi, Kazuaki Ishihara
Science 2014, 345(6194), 291–294.
DOI: 10.1126/science.1254976
http://www.sciencemag.org/content/345/6194/291.abstract
Perspective
Creating antioxidants by oxidation catalysis
Boris J. Nachtsheim
Science 2014, 345(6194), 270-271
DOI: 10.1126/science.1257347
http://www.sciencemag.org/content/345/6194/270.summary


▼Cooperative Activation with Chiral Nucleophilic Catalysts and N-Haloimides: Enantioselective Iodolactonization of 4-Arylmethyl-4-pentenoic
Acids
Hidefumi Nakatsuji, Yasuhiro Sawamura, Akira Sakakura, Kazuaki Ishihara*
Angew. Chem., Int. Ed. 2014, 53(27), 6974–6977
▷DOI: 10.1002/anie.201400946
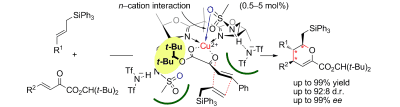
▼Catalytic Enantioselective Inverse Electron Demand Hetero-Diels–Alder Reaction with Allylsilanes
Yuki Matsumura, Takahiro Suzuki, Akira
Sakakura, Kazuaki Ishihara*
Angew. Chem., Int. Ed. 2014, published online 29 Apr 2014.
▷DOI: 10.1002/anie.201402934
▼The Enantioselective Diels–Alder Reaction of 1,2-Dihydropyridines with α-Acyloxyacroleins Catalyzed by Chiral Primary Ammonium
Salt
Kazuaki Ishihara*, Hiroki Yamada, Matsujiro Akakura
Chem. Commun. 2014, published online 11 Apr 2014.
▷DOI: 10.1039/C4CC01445F

▼著者担当したG. A. Molander, P. Knochel編, Comprehensive Organic Synthesis, 2nd Edition, Vol. 6 (Elsevier)が今春出版
“5.9 Intermolecular Diels–Alder Reactions”
Kazuaki Ishihaira, Akira Sakakura
In: Gary A. Molander and Paul Knochel (eds.), Comprehensive Organic Synthesis, 2nd Edition, Vol. 5, Oxford: Elsevier; 2014, pp. 351-408.
“5.10 Hetero-Diels–Alder Reactions”
Kazuaki Ishihaira, Akira Sakakura
In: Gary A. Molander and Paul Knochel (eds.), Comprehensive Organic Synthesis, 2nd Edition, Vol. 6, Oxford: Elsevier; 2014, pp. 409–465.
“6.14 Functional Group Transformations via Carbonyl Dervatives”
Muhammet Uyanik, Kazuaki Ishihaira
In: Gary A. Molander and Paul Knochel (eds.), Comprehensive Organic Synthesis, 2nd Edition, Vol. 5, Oxford: Elsevier; 2014, pp. 573–597.
▼総合論文 “ハロゲンをLewis酸中心として活用する高選択的有機変換反応の開発”
石原 一彰
有機合成化学協会誌 2014, 72(2), 137–148.
(2012年度有機合成化学協会企業冠賞 第一三共・創薬有機化学賞受賞) (2014年2月1日発行)


▼Selective Bromocyclization of 2-Geranylphenols Promoted by Phosphite–Urea Cooperative Catalysts
Yasuhiro Sawamura,
Hidefumi Nakatsuji, Matsujiro Akakura, Akira Sakakura*, Kazuaki Ishihara*
Chirality 2014, 26, 356–360.
▷DOI: 10.1002/chir.22297

Nucleophilic phosphite–urea cooperative catalysts are highly efficient for the bromonium-induced cyclization of 2-geranylphenols. Phosphite–N,N’-dimethylurea catalysts also show moderate activity, probably due to the steric effect of their bent conformation.
▼Chiral 1,1′-Binaphthyl-2,2′-Disulfonic Acid (BINSA) and Its Derivatives for Asymmetric
Catalysis
Manabu Hatano, Kazuaki Ishihara*
Asian J. Org. Chem. 2014, 3, 352-365.
▷DOI: 10.1002/ajoc.201300256
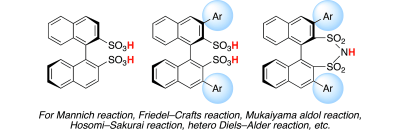
Chiral Brønsted acid catalysts, which are derived from commercially available (R)- or (S)- 1,1′-bi-2-naphthol (BINOL), have found widespread application as chiral organocatalysts and chiral ligands for metal species. The Brønsted acidity of these catalysts is considered to be associated with their catalytic activity. Therefore, due to the rapid development of asymmetric organocatalysis, chiral 1,1′-binaphthyl-2,2′-disulfonic acid (BINSA) and the corresponding chiral binaphthyl disulfonimides are highly attractive as stronger chiral Brønsted acid catalysts than carboxylic acids, phosphoric acids, and phosphoramides. This Focus Review summarizes the latest achievements in chiral BINSA chemistry, particularly in the field of asymmetric organocatalysis.
▼寄稿論文「キラルビナフチルジスルホン酸を鍵とする分子触媒設計の新機軸」
TCIメール 2014, 1, No. 160, 2-23
波多野学、西川圭祐、石原 一彰
無料でダウンロードできます。
http://www.tcichemicals.com/ja/jp/support-download/tcimail/backnumber/article/160dr.pdf
英語版
▼Innovative Molecular Design of Chiral 1,1′-Binaphthyl 2,2′-Disulfonic Acid (BINSA)
TCIMAIL 2014, 1, No. 160, 2-16
http://www.tcichemicals.com/en/ap/support-download/tcimail/backnumber/article/160Edr.pdf









