論文 2012

▼酵素を凌駕するテーラーメイド型配座柔軟性キラル超分子触媒の精密設計
波多野 学*
有機合成化学協会誌 2012, 70(12), 1242–1254.
付記:【十字路】テーラーメイド触媒・レディメイド触媒
波多野 学*
有機合成化学協会誌 2012, 70(12), 1307.

▼Conformationallyflexible chiral hypervalent Organoiodine catalysts for enantioselective Oxidative Transformations
Muhammet Uyanik
Kazuaki Ishihara*
J. Synth. Org. Chem., Jpn. 2012, 70(11), 1116–1122.
▷https://www.jstage.jst.go.jp/browse/yukigoseikyokaishi/70/11/_contents/-char/ja/

▼テーラーメイド型超分子触媒の設計
波多野 学
石原 一彰*
ケミカルエンジニヤリング 2012, 57(9), 656-661.
▷http://www.kako-sha.co.jp/volchem.html
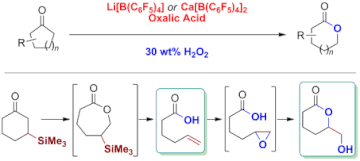
▼Baeyer-Villiger Oxidation and Oxidative Cascade Reactions with Aqueous Hydrogen Peroxide Catalyzed by Lipophilic Li[B(C6F5)4] and Ca[B(C6F5)4]
Muhammet Uyanik
Daisuke Nakashima
Kazuaki Ishihara*
Angew. Chem. Int. Ed. 2012, 51(36), 9093-9096.
Article first published online: 15 AUG 2012
Efficient and selective: Two lipophilic catalysts were used for Baeyer–Villiger (BV) oxidations to give lactones in high yields (see scheme). Cascade reactions involving this BV oxidation were used to selectively obtain either unsaturated carboxylic acids or hydroxylactones in high yields from β-silyl cyclohexanones.

▼In Situ Generated “Lanthanum(III) Nitrate Alkoxide” as a Highly Active and Nearly Neutral Transesterification Catalyst
Manabu Hatano
Sho Kamiya
Kazuaki Ishihara*
Chem. Commun. 2012, 48(76), 9465-9467.
In situ generated lanthanum(III) nitrate alkoxide is a highly active and nearly neutral transesterification catalyst, which can promote non-epimerized transesterification of α-substituted chiral carboxylic esters under reflux conditions.

▼α-Heterosubstituted beta-Alkylacroleins as Useful Multisubstituted Dienophiles for Enantioselective Diels-Alder Reactions
Akira Sakakura
Hiroki Yamada
Kazuaki Ishihara*
Asian. J. Org. Chem. 2012, 1(2), 133-137.
Building complexity: α-(N,N-Diisopropylcarbamoyloxy)-β-alkylacroleins and α-(acylamino)crotonaldehydes were synthesized as useful multisubstituted dienophiles. The Diels–Alder reaction of these dienophiles catalyzed by organoammonium salts provides an efficient route to structurally complicated chiral building blocks that contain a quaternary carbon atom substituted with an oxygen- or nitrogen-containing moiety.
■Inside Coverに採用された

▼IBS-Catalyzed Regioselective Oxidation of Phenols to 1,2-Quinones with Oxone
Muhammet Uyanik
Tatsuya Mutsuga
Kazuaki Ishihara*
Molecules 2012, 17(7), 8604-8616.
(the Special Issue: Hypervalent Compounds)
We have developed the first example of hypervalent iodine(V)-catalyzed regioselective oxidation of phenols to o-quinones. Various phenols could be oxidized to the corresponding o-quinones in good to excellent yields using catalytic amounts of sodium salts of 2-iodobenzenesulfonic acids (pre-IBSes) and stoichiometric amounts of Oxone as a co-oxidant under mild conditions. The reaction rate of IBS-catalyzed oxidation under nonaqueous conditions was further accelerated in the presence of an inorganic base such as potassium carbonate (K2CO3), a phase transfer catalyst such as tetrabutylammonium hydrogen sulfate (nBu4NHSO4), and a dehydrating agent such as anhydrous sodium sulfate (Na2SO4).

▼N,N-Diarylammonium Pyrosulfate as a Highly Effective Reverse Micelle-Type Catalyst for Hydrolysis of Esters
Yoshiki Koshikari
Akira Sakakura
Kazuaki Ishihara*
Org. Lett. 2012, 14(12), 3194-3197.
Reverse micelle-type N,N-diarylammonium pyrosulfate (3-5 mol %) efficiently catalyzes the hydrolysis of esters (up to 100 mmol scale) under organic solvent-free conditions. The present method is successfully applied to the hydrolysis of various esters without the decomposition of the base-sensitive moieties and without any loss of optical purity for alpha-heterosubstituted carboxylic acids.

▼Enantioselective Diels-Alder Reaction of alpha-(Acylthio)acroleins: A New Entry to Sulfur-Containing Chiral Quaternary Carbons
Akira Sakakura
Hiroki Yamada
Kazuaki Ishihara*
Org. Lett. 2012, 14(12), 2972-2975.
A catalytic and enantioselective Diels-Alder reaction of alpha-(carbamoylthio)acroleins induced by an organoammonium salt of chiral triamine is described. alpha(Carbamoylthio)acroleins are designed and synthesized as new sulfur-containing dienophiles for the first time. The Diels-Alder reaction affords chiral tertiary thiol precursors with up to 91% ee.
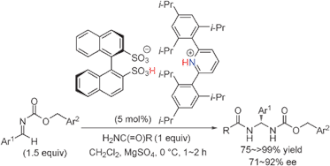
▼Enantioselective direct aminalization with primary carboxamides catalyzed by chiral ammonium 1,1'-binaphthyl-2,2'-disulfonates
Manabu Hatano
Takuya Ozaki
Yoshihiro Sugiura
Kazuaki Ishihara*
Chem. Commun. 2012, 48, 4986-4988.
A highly effective catalytic enantioselective direct aminal synthesis was developed. Chiral ammonium 1,1'-binaphthyl-2,2'-disulfonates, which were prepared in situ from (R)-BINSA and achiral amines, promoted the enantioselective addition of primary amides to aromatic aldimines.
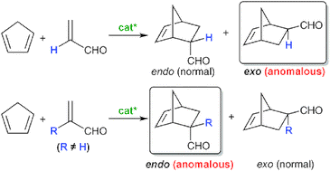
▼Conformationally Flexible Chiral Supramolecular Catalysts for Enantioselective Diels-Alder Reactions with Anomalous Endo/Exo Selectivities
Manabu Hatano
Kazuaki Ishihara*
Chem. Commun. 2012, 48(36), 4273-4283.
The potential of supramolecular catalysts to realize anomalous regio- and/or stereoselectivity in organic synthesis is highly attractive. To date, there have been a few examples of non-polymeric and non-covalent chiral supramolecular catalysts that induce practical enantioselectivity. In this regard, a metal-organic framework (MOF) may be one of the most important techniques for constructing conformationally rigid supramolecular catalysts. However, it is not easy to use the MOF technique to fine-tune a much more precise cage in catalysts for anomalous purposes. To establish high catalytic activity with anomalous regio- and/or stereoselectivity, in principle, an artificial cage should be conformationally flexible, like an active pocket in an enzyme with an induced-fit function. In this feature article, we focus on the anomalous endo/exo-selective Diels-Alder reaction, and overview the development of the successive catalysts including our recent highly active, conformationally flexible, and chiral supramolecular catalysts. The evolution from ‘ready-made’ single-molecule catalysts to ‘tailor-made’ supramolecular catalysts could offer not only high enantioselectivity but also high anomalous endo/exo-selectivities due to substrate-specific characteristics, as with enzymes.
波多野 学
石原 一彰*
環境に負荷をかけることなく,安全かつ安価に,大量に目的の有機化合物を合成できる反応技術開発は,ますます重要な研究課題になっている。言うまでもなく,エステル合成は工業化学の基幹産業のひとつである。それゆえ激しい国際競争によるトップレベルの経済性と安全性に加え,今なおそれを推進する新しい技術力が求められている。著者らはその一端を担うべく,カルボン酸エステルの実用的な触媒的合成法の確立を目的に,ランタン(III)イソプロポキシドとジエチレングリコールモノメチルエーテルよりin situで簡便に調製される均一系高活性触媒を用いるエステル交換反応を開発した。本法を用いて,等モル量の第1-3級アルコールからカルボン酸エステル,炭酸エステル,カルバミン酸エステルの効率的合成を実現した。
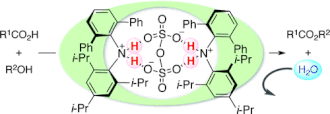
▼Hydrophobic N,N-Diarylammonium Pyrosulfates as Dehydrative Condensation Catalysts under Aqueous Conditions
Akira Sakakura
Yoshiki Koshikari
Matsujiro Akakura
Kazuaki Ishihara*
Org. Lett. 2012, 14(1), 30-33.
Oil-soluble N,N-diarylammonium pyrosulfates as nonsurfactant-type catalysts for the dehydrative ester condensation under aqueous conditions are described. Preheat treatment of dibasic sulfuric acid with bulky N,N-diarylamines generates water-tolerant salts of pyrosulfuric acid as active catalyst species. The present catalysts in water can also widely be applied to unusual selective esterifications and dehydrative glycosylation.

▼2-Iodoxy-5-Methylbenzenesulfonic Acid-Catalyzed Selective Oxidation of 4-Bromobenzyl Alcohol to 4-Bromobenzaldehyde or 4-Bromobenzoic Acid with Oxone
Muhammet Uyanik
Kazuaki Ishihara*
Org. Synth. 2012, 89, 105-114.
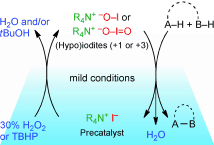
▼Catalysis with In Situ-Generated (Hypo)iodite Ions for Oxidative Coupling Reactions
Muhammet Uyanik
Kazuaki Ishihara*
ChemCatChem 2012, 4(2), 177-185.
(Article first published online: 23 DEC 2011)
Hyping up the iodines: The discovery and development of oxidative coupling reactions catalyzed by inorganic iodine species (i.e., hypoiodite or iodite), which are generated in-situ from an iodide ion and hydrogen peroxide or tert-butyl hydroperoxide as an environmentally benign oxidant is highlighted.









